Heart Failure patients have a 50% 5-year survival rate
Heart Failure patients with preserved ejection fraction (HFpEF) account for ~50% of all patients1
There are limited
therapies for treating
patients with HFpEF
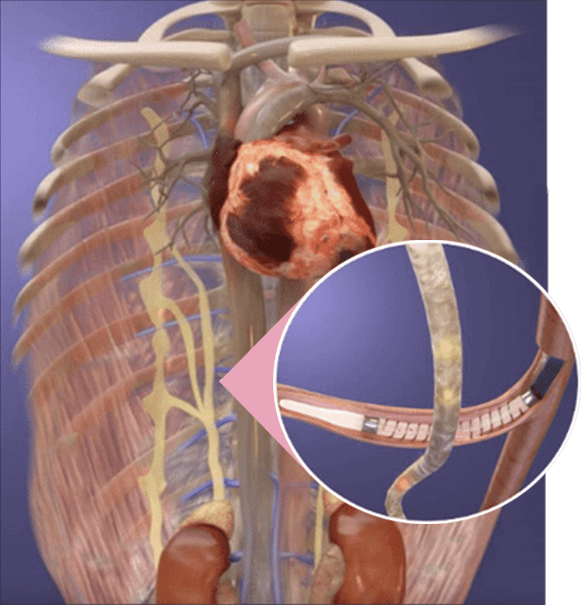
A hallmark of heart failure progression is an overactive sympathetic nervous system (SNS). The SAVM procedure aims to calm sympathetic nerve activity and address a root cause of heart failure.
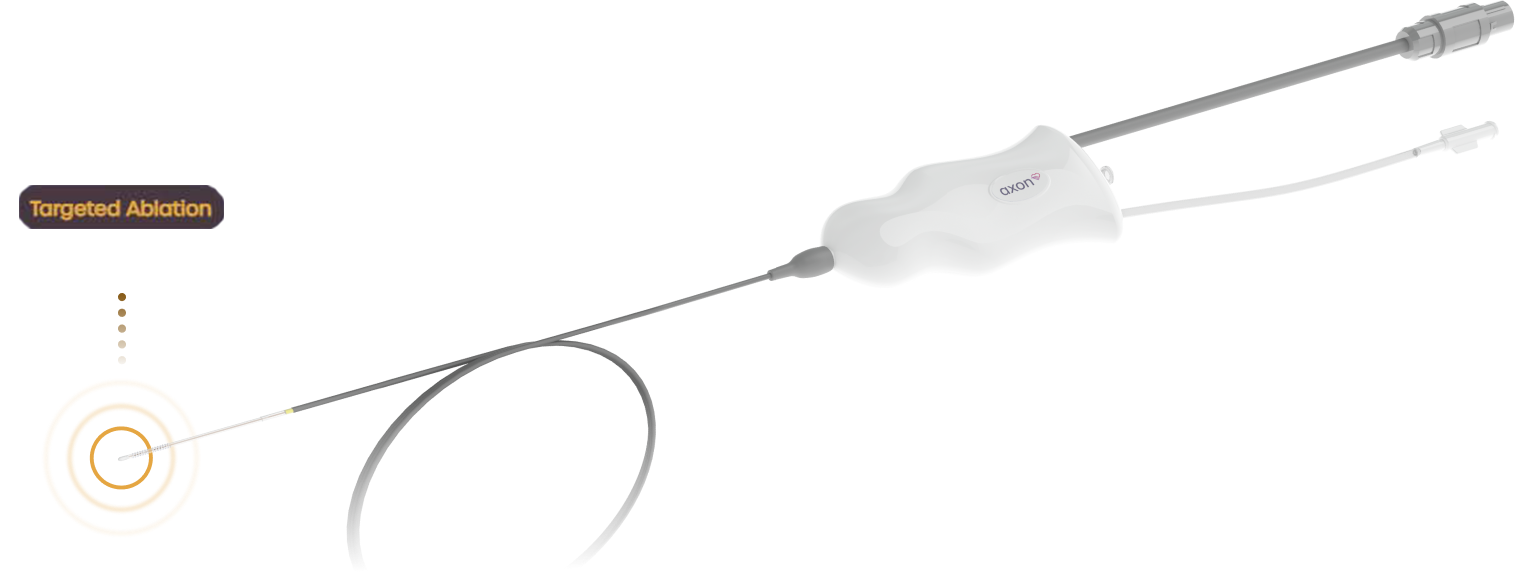
During the implant-free procedure, the Axon Ablation Catheter is inserted into the femoral vein, navigated through the venous system to a target vessel near the right greater splanchnic nerve.
Once there, the vessel is treated with targeted ablation therapy to block nerve signals, diminishing sympathetic activity and normalizing volume balance in the body.
The outpatient procedure can typically be performed in under an hour.
1 Fudim M et al. J Am Heart Assoc. 2017 Aug 17;6(8)